|
Protocol
for Chemiluminescence Labeling
1.
INTRODUCTION
Assay
Designs' Sub-Attomole Labeling Kit, combined with
Turner BioSystems TD-20/20 Luminometer, gives rise
to rapid attachment and analysis of chemiluminescent
Acridinium Esters to lysine groups on most antibodies,
proteins, nucleic acids and some peptides. As an alternative
to 125I labels, the Acridinium Esters can
be used to label antigens for competitive immunoassays,
or signal antibodies for immunometric assays. In addition,
chemiluminescent-labeled nucleic acids may be used
as reporter molecules in DNA probe based assay systems.
Labeling, purifying, and then detecting the labels
on the TD-20/20 Luminometer can be achieved in less
than 60 minutes.
2.
MATERIALS REQUIRED
From Turner BioSystems:
- TD-20/20
Luminometer with Dual Injectors (P/N 2020-050)
- 12
mm Test Tube Holder and
- 12
mm x 50 mm Polypropylene Test Tubes (P/N 2020-911)
From
Assay Designs:
- Sub-Attomole
Labeling Kit (Cat. No. 90701) contains enough material
to perform up to 5 labeling experiments equivalent
to 1 mg of IgG each and includes the following materials:
- Acridinium
Ester with a N-hydroxysuccinimide Ester group, 100
mg
- Dry
Dimethyl Formamide, 0.5 ml (for dissolving the Acridinium
Ester)
- Bicarbonate
Labeling Buffer(for diluting the antibody, protein
or nucleic acid)
- 10%
Solution of Lysine (to stop the labeling reaction)
- Gel
Filtration Column (for separation of the labeled
product from excess Acridinium Ester)
- Column
Buffer 10x Concentrate, 100 ml
- Set
of Trigger Solutions, 30 ml each (for detection
of the labeled antibody, protein or nucleic acid)
NOTE:
The Acridinium Ester supplied in this kit has an N-hydroxysuccinimidyl
(NHS) ester-labeling group attached to a 2-carbon
spacer arm. The NHS ester-labeling group will covalently
attach to any primary amino group on the protein,
peptide or nucleic acid to be labeled. At pH's above
neutral, the NHS ester labeling group is subject to
nucleophilic attack by the amine group on the protein,
peptide or nucleic acid. As a result, the NHS group
is displaced from the Acridinium Ester to form a stable
amide bond between the Acridinium Ester and the protein,
peptide or nucleic acid.
In addition, the sample to be labeled must be free
of all aliphatic primary amines and free of all preservatives.
Samples should not contain Tris based buffers, sodium
azide, or other nucleophilic materials. Salt free
lyophilized Preparations or concentrated solutions
are ideal. These can be diluted in the Bicarbonate
Labeling Buffer to achieve optimal pH for labeling.
3.
INSTRUMENT SET-UP
3.1
Turn on TD-20/20 Luminometer and warm up for 5 minutes.
3.2 Place sample adaptor and holder into sample
chamber.
3.3 Prime (at least 4 times) injector 1 with
Trigger Solution 1 and injector 2 with Trigger Solution
2. Set injection volumes to 100 µl on each injector.
Refer to your operating manual for further details
on priming injectors and setting injection volumes.
3.4 Set parameters of instrument: Delay=0,
Integrate Time=4 seconds, Reps=1. Refer to your operating
manual for further details on how to set parameters.
3.5 Set Mode to DLR by pressing [SETUP][2], and
then Toggle to select DLR. Press [ESC] when done.
4. LABELING PROCEDURE
4.1
Allow the diluted Peptide, Protein or Nucleic Acid
to come to room temperature. If the material to be
labeled is lyophilized, add sufficient Labeling Buffer
to make a 5 mg/ml solution.
4.2 Add the calculated volume of the Acridinium
Ester solution in DMF to the peptide, protein or nucleic
acid solution and vortex. Stir at room temperature
for 30 minutes.
4.3 After 30 minutes, add 10 µl of the 10%
lysine solution to stop any further reaction and vortex.
Stir at room temperature for 15 minutes.
4.4 Apply the labeled sample to the top of
the washed, drained column. Let the sample enter the
column bed. With a clean pipet tip, add
800 µl of column buffer to the reaction tube and vortex
to mix. Apply this to the column. With a clean pipet
tip, follow with 1 ml aliquots of the column buffer.
Collect 1 ml fractions. The column will stop draining
automatically after each 1 ml aliquot is added. Collect
12-14 fractions.
5. ANALYZING SAMPLES
5.1
Pipet 1 µl of each fraction into numbered duplicate
12 mm x 50 mm test tubes.
5.2 Insert test tube in the luminometer and
press [GO]. The luminometer will inject Trigger Solution
1, take a reading, inject Trigger Solution 2, and
then take another reading. It will also calculate
the ratio between the two readings. You will only
be concerned with the second reading, when both Trigger
Solutions have been added to the sample.
5.3 Pool the first fraction(s) that contain
light activity. The pooled sample(s) should be aliquoted
and stored at -20°C.
6.
EXAMPLE OF TYPICAL RESULTS
Graph
1 below is an example of the Acridinium Ester labeling
of mouse IgG. The graph shows the total Relative Light
Units (RLU) obtained from the TD-20/20 Luminometer
for 1 m l of each fraction. The total RLU includes
any dilutions that were performed.
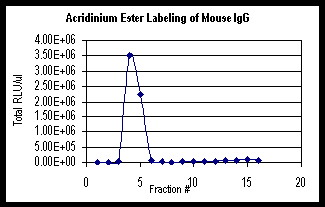
Graph
1: Acridinium Ester Labeling of mouse IgG using
Turner BioSystems TD-20/20 Luminometer.
7. REFERENCE INFORMATION
7.1
The uses of Acridinium Ester labeled antibodies, antigens
and DNA have been described in a number of publications.
These references are available upon request from Assay
Designs by phones at (734) 668-6113, by fax (734)668-2793,
or by Email at TechServ@assaydesigns.com.
8.
GENERAL USES
8.1
Streptavidin, progesterone, thyroxin and free thyroxin,
alpha-fetoprotein, thyroid stimulating hormone, human
chorionic gonadotropin, human complement component
C9, staphylococcal peptidoglycan, carcinoembryonic
antigen, Chlamydia trachomatis and Neisseria gonorrhea
RNA have all been detected using this molecule or
analogs. Invariably the assays developed using this
material show greater sensitivity, speed of analysis,
dynamic range and/or decreased sample volume.
9.
SPECIAL THANKS
Turner
BioSystems would like to express their appreciation
to Assay Designs for their help in providing much
of the information in this applications note.
Turner BioSystems would also like to express their
appreciation to Teresa Yang of Sentinel Biosciences
for providing the graph in Section 6.
10.
ABOUT ASSAY DESIGNS, INC.
Orders
for Assay Designs' products may be placed by:
Phone:
(800) 833-8651 (U.S. or Canada)
(734) 668-6113 (local or international)
Fax: (734) 668-2793
E-mail: info@assaydesigns.com
Internet: http://www.assaydesigns.com
Mailing Address (HQ):
Assay Designs, Inc.
1327 Jones Drive
Ann Arbor, MI 48105
|

