|
Protocol
for Quantiation of Proteins using NanoOrange™
1.
Introduction
Fluorometric
quantitation of proteins in solution using the Turner
BioSystems TD-700 Laboratory Fluorometer and Molecular
Probes' NanoOrange™ Protein Quantitation Kit offers
an unprecedented combination of convenience and sensitivity.
Protein concentrations as low as 10 ng/mL can be measured.
This level of sensitivity is much superior to spectrophotometric
techniques such as the BCA method (0.5 µg/mL), the Bradford
assay (1 µg/mL), the Lowry assay (1 µg/mL), or 280 nm
absorbance (50 µg/mL). (1-4)The NanoOrange™ assay
also shows less protein-to-protein variability than
the Bradford assay.
To
perform a protein assay, the protein sample is simply
added to the NanoOrange™ reagent in a specialized
diluent and this mixture is heated at 95° C for ten
minutes. Fluorescence can be measured as soon as the
mixture has cooled to room temperature. Alternatively,
samples can be read up to six hours after preparation
with no loss in sensitivity, as long as samples are
protected from light. The NanoOrange™ reagent is
virtually nonfluorescent in aqueous solution, becoming
strongly fluorescent at about 570—590 nm upon interaction
with proteins, when excited at about 470—490 nm.
Detection of the fluorescence using the TD-700 fluorometer
equipped with a fluorescein filter kit allows protein
concentrations from 10 ng/mL to 10 µg/mL to be accurately
measured relative to a standard curve (Figures 1 and
2).
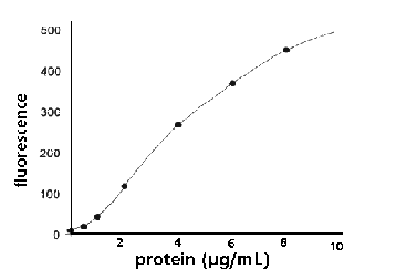
Figure 1. Full-range calibration plot for bovine
serum albumin (BSA) using the TD-700 Fluorometer and
the NanoOrange™ Protein Quantitation Kit.
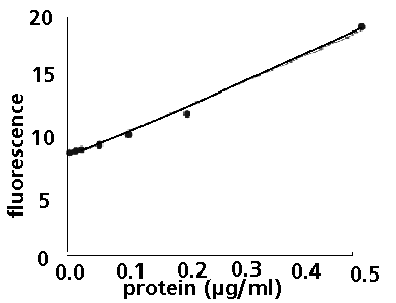
Figure 2. Low-range calibration plot for bovine serum
albumin (BSA) using the TD-700 Fluorometer and the NanoOrange™
Protein Quantitation Kit.
2.
Materials Required
- TD-700
Fluorometer with standard PMT and 10 x 10 mm square
cuvette adaptor (P/N 7000-009)
- Fluorescein
filter kit (P/N 10-086R) which includes 486 nm excitation
filter (P/N 034-0486), 510-700 nm emission filter
(P/N 10-109R-C), and two Blue Mercury Vapor Lamps
(P/N 10-089).
- 10
x 10 mm square polystyrene disposable cuvettes (P/N
7000-957)
- NanoOrange™
Protein Quantitation Kit, supplied by Molecular Probes,
Inc., Eugene, Oregon, catalog number N-6666. The kit
contains 1.0 mL NanoOrange™ protein quantitation
reagent (500X concentrate), 50 mL NanoOrange™
protein quantitation diluent (10X concentrate), and
0.5 mL bovine serum albumin (BSA) standard (2 mg/mL).
The kit contents are sufficient for 200 assays using
a 2 mL volume in a standard cuvette. Handling, storage,
and the use of the reagents should be performed in
accordance with the product information sheet supplied
by Molecular Probes, Inc.
3.
Experimental Protocol
3.1
Reagent Preparation
Dilute
the concentrated NanoOrange™ protein quantitation
diluent 10-fold in distilled water. For each assay,
2.5 mL of 1X protein quantitation diluent will be
required. Just prior to running the experiment,
dilute the NanoOrange™ protein quantitation
reagent 500-fold into the 1X protein quantitation
diluent.
For
example, to prepare 50 mL of 1X NanoOrange™
working solution (enough for 20 assays), first prepare
the 1X diluent by mixing 5 mL of the 10X diluent
stock with 45 mL of distilled water; next add 100
µL of the NanoOrange™ reagent and mix thoroughly.
Protect the 1X NanoOrange™ working solution
from photodegradation by storing it in an opaque
bottle, covering it with foil or placing it in the
dark. For best results, the working solution
should be used within a few hours of its preparation.
3.2
Protein Standard Curve
A
standard curve should be generated for converting
sample fluorescence into protein concentration.
Ideally, the protein type used for the standard
curve should be the same as that which is used in
the experiment; however, as with other protein assays,
bovine serum albumin (BSA) serves as a convenient
reference standard. The NanoOrange™ Kit includes
a 2 mg/mL sample of BSA that can be used to prepare
a standard curve. To serve as an effective control,
the protein solution used to prepare the standard
curve should also contain levels of contaminants
similar to those present in the experimental samples
[note A]. The reference standard curve is used not
only to convert fluorescence to protein concentration,
but also to control for any day-to-day readout variation
of the fluorometer. The standard curve may be generated
to cover the full assay range, 0-10 µg/mL, or to
cover a selected range. This section describes how
to generate a simple standard curve with points
corresponding to 0, 1, 3, 6 and 10 µg BSA per mL.
If desired, serial dilutions can be made to create
additional standards ranging from 0.01 to 0.6 µg/mL,
to fill out the standard curve in the low range.
3.2.1
Prepare a 10 µg/mL solution of BSA by diluting 30
µL of the BSA standard into 5.97 mL of the 1X NanoOrange™
working solution prepared in section 3.1.
3.2.2
Dilute the 10 µg/mL BSA solution to make 0, 1, 3,
6 and 10 µg/mL standards, as described in Table
1. If desired, prepare 0.1, 0.3 and 0.6 µg/mL standards,
as described in Table 1, by diluting a 1 µg/mL BSA
solution [note B]. Prepare the 1 µg/mL BSA solution
by diluting 300 µL of 10 µg/mL BSA (made in section
3.2.1) into 2.70 mL of 1X NanoOrange™ working
solution.
3.2.3
If desired, prepare 0.01, 0.03 and 0.06 µg/mL standards,
as described in Table 1, by diluting a 0.1 µg/mL
BSA solution [note B]. Prepare the 0.1 µg/mL BSA
solution by diluting 300 µL of 1 µg/mL BSA (made
in section 3.2.2) into 2.70 mL of 1X NanoOrange™
working solution.
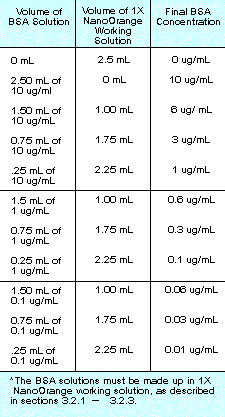 Table
1. Protocol for preparing a standard curve using
BSA. Table
1. Protocol for preparing a standard curve using
BSA.
3.2.4
Incubate samples at 90° C to 96° C for 10 minutes,
protected from light. After heating, cool to room
temperature for 20 minutes, protected from light.
3.2.5
After cooling, transfer 2.0 mL [note B] of the
sample to a standard acrylic fluorescence cuvette
and measure the fluorescence using the TD-700 Fluorometer
installed with the blue mercity vapor lamp (P/N
10-089), excitation filter 034-0486, and emission
filter 10-109R-C. Insert the most fluorescent sample
first (10 µg/mL protein) and calibrate the instrument
sensitivity as directed in the TD-700 manual (press
#2, calibrate). This procedure automatically optimizes
the instrument sensitivity to match the fluorescence
of the sample.
3.2.6
Measure the fluorescence of the remaining samples.
To equalize any photobleaching effects, insert
samples into the fluorometer for approximately equal
time periods. The fluorescence value of the
reagent blank (0 µg/mL protein) may be subtracted
from that of each sample. Corrected or uncorrected
data may be used to generate a standard curve of
fluorescence versus protein concentration (for example,
see Figures 1 and 2).
3.3
Sample Analysis
3.3.1
Dilute the experimental protein solution in 1X NanoOrange™
working solution (prepared in section 3.1) to achieve
a final volume of 2.5 mL [note B]. You may wish
to use two or three different dilution factors for
a given sample. Higher dilution factors will diminish
levels of contaminants [note A]; however, extremely
small sample volumes should be avoided as they are
difficult to pipet accurately.
3.3.2
Incubate samples at 90° C to 96° C for 10 minutes,
protected from light. After heating, cool to room
temperature for 20 minutes, protected from light.
3.3.3
After cooling, transfer 2.0 mL [note B] of the sample
to a standard acrylic fluorescence cuvette and measure
the fluorescence using the same instrument parameters
as used in generating the standard curve (section
3.2.6). To equalize any photobleaching effects,
insert samples into the fluorometer for similar
time periods to those used for the standard curve
measurements.
3.3.4
If the standard curve was plotted using blank-subtracted
data (section 3.2.6), the reagent blank (0 µg/mL
protein) fluorescence value must also be subtracted
from that of each of the samples. Determine the
protein concentration of the sample from the standard
curve generated in section 3.2.6.
4.
Footnotes
[A]
Various compounds known to contaminate protein preparations,
including salts, detergents and reducing agents may
interfere with the NanoOrange™ protein quantitation
assay. Protein standard and blank samples should be
prepared in solutions that match the composition of
the unknown samples as closely as possible. The maximum
tolerable concentrations for avoiding appreciable
interference are approximately 10 mM for salts (including
ammonium sulfate), 100 mM for reducing agents (DTT
and b-mercaptoethanol) and 0.01% (w/v) for SDS. For
other detergents (Tween®-20 and Triton® X-100), the
tolerance level is lower (0.001% (w/v)). See Molecular
Probes' product information sheet MP6666 for further
details.
[B]
Pipetting and sample handling are the largest sources
of experimental error in the assay. Accurate volume
measurements are essential when making up and transferring
samples.
5.
Warnings and Precautions
The
NanoOrange™ Protein Quantitation Reagent is the
subject of patent applications filed by Molecular
Probes, Inc. and is not available for commercial uses
without a specific agreement from Molecular Probes,
Inc. NanoOrange™ is a trademark of Molecular
Probes, Inc. Triton is a registered trademark of Rohm
& Haas, Inc. Tween is a registered trademark of
ICI Americas, Inc.
6.
References
- Anal
Biochem 150, 76 (1985)
- Anal
Biochem 72, 248 (1976)
- J
Biol Chem 193, 265 (1951)
- Scopes,
R.K., Protein Purification, Principles and Practice,
2nd Edition, Springer-Verlag (1987)
|

